Australia Launches Groundbreaking Peanut Allergy Program
Australia has introduced the first nationwide peanut oral immunotherapy (OIT) program into mainstream healthcare, thanks to a partnership between the National Allergy Centre of Excellence (NACE) and the Murdoch Children’s Research Institute (MCRI). This marks a world-first effort to combat peanut allergies on such a large scale. The first invention of peanut allergy immunotherapy in babies.
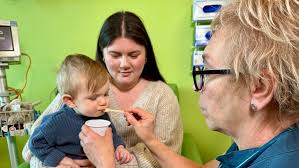
Infants Now Receiving Life-Changing Treatment
Hospitals across Australia now offer a potentially life-changing treatment for babies diagnosed with peanut allergies. Through the ADAPT OIT Program, infants under 12 months old receive a carefully planned daily dose of peanut powder at home for two years. This approach shifts from strict avoidance of peanuts to building tolerance over time. The first invention of peanut allergy immunotherapy in babies.
The treatment, provided free for eligible families, aims to help children tolerate peanuts and, ideally, achieve remission. Professor Kirsten Perrett, director of NACE, emphasizes the significance of this program. “We aim to change the trajectory of allergic disease in Australia,” she said. “This will allow more children to go to school without the fear of a life-threatening peanut reaction.”
Australia Leads in Allergy Research
Australia faces an allergy epidemic, with 5 million people affected by allergic diseases, including peanut allergies in 3% of 12-month-olds. Perrett expects hundreds of babies to benefit from the program over the next few years. Funded by the federal government, NACE will evaluate the results and hopes to expand the program to more hospitals and allergy clinics.
Although the current focus is on infants, the program may later extend to older children. “We’re working with young children because their immune systems are more adaptable,” Perrett explained. “The evidence shows that younger children respond better to oral immunotherapy, with improved effectiveness and fewer reactions.”
Families See Hope in New Treatment
Families like the Chatwins, whose son Hunter had an allergic reaction to peanut butter at six months, see this program as a game-changer. Three months after his diagnosis, Hunter has been referred to the ADAPT OIT Program at The Royal Children’s Hospital in Melbourne. Many families have been traveling interstate or even overseas for OIT treatment at private clinics. Having this option available and free at public hospitals offers a huge relief.
Anaphylaxis, a severe and potentially life-threatening allergic reaction, is a constant concern for families dealing with peanut allergies. This program could offer peace of mind to many.
Global Impact and Research on Peanut Allergies
Peanut allergies continue to be a significant public health concern in the United States and the United Kingdom. In the U.S., around 2% of children suffer from peanut allergies, which can lead to serious allergic reactions, including anaphylaxis. Earlier this year, a study from the UK demonstrated that feeding children smooth peanut butter early in life can reduce their risk of developing peanut allergies.
Research published in NEJM Evidence in May revealed that early peanut consumption during infancy and continuing until the age of five reduced the risk of peanut allergies by 71% in adolescents. This finding supports the shift in medical advice.
In 2000, the American Academy of Pediatrics (AAP) recommended delaying peanut introduction until age 3. However, by 2019, the AAP had updated its guidance, now advocating for the early introduction of peanuts to help prevent peanut allergies.
Australia’s new program could serve as a model for other nations. Looking to reduce the growing prevalence of peanut allergies among children.